Yunsung F&C, growing based on quality and technology.
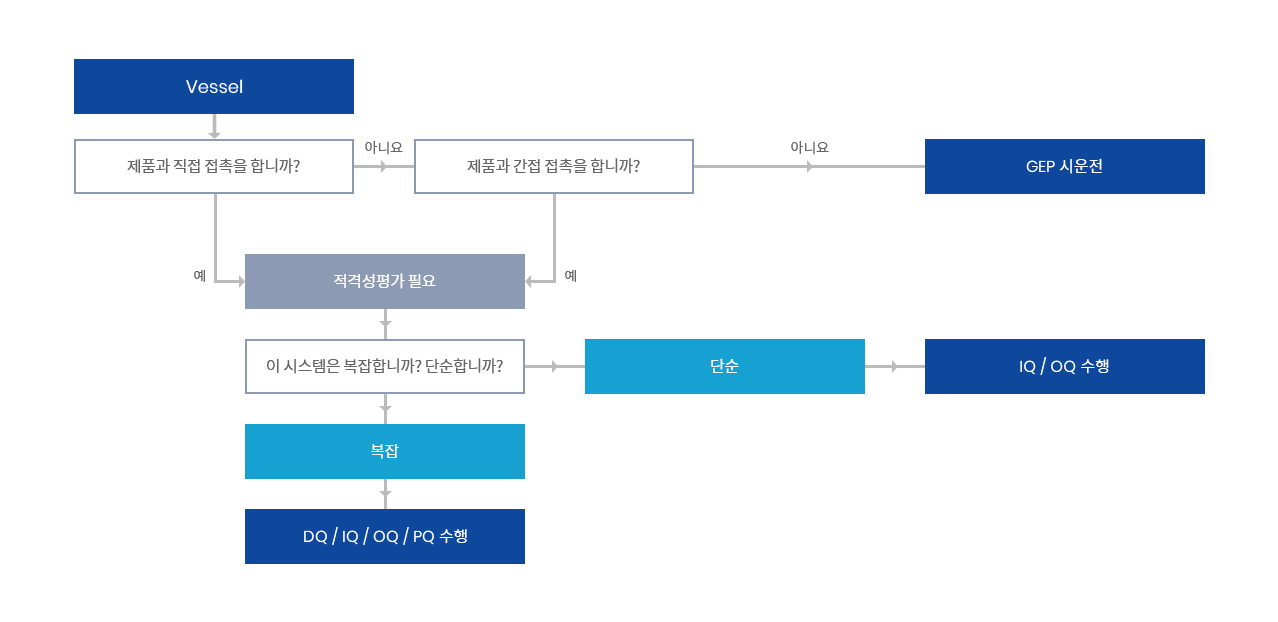
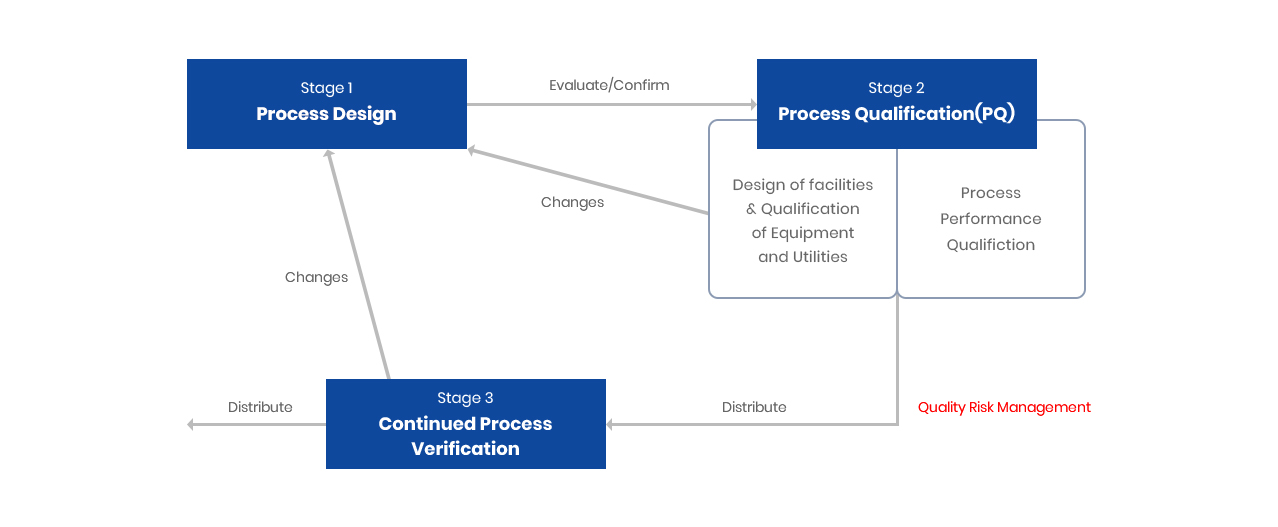
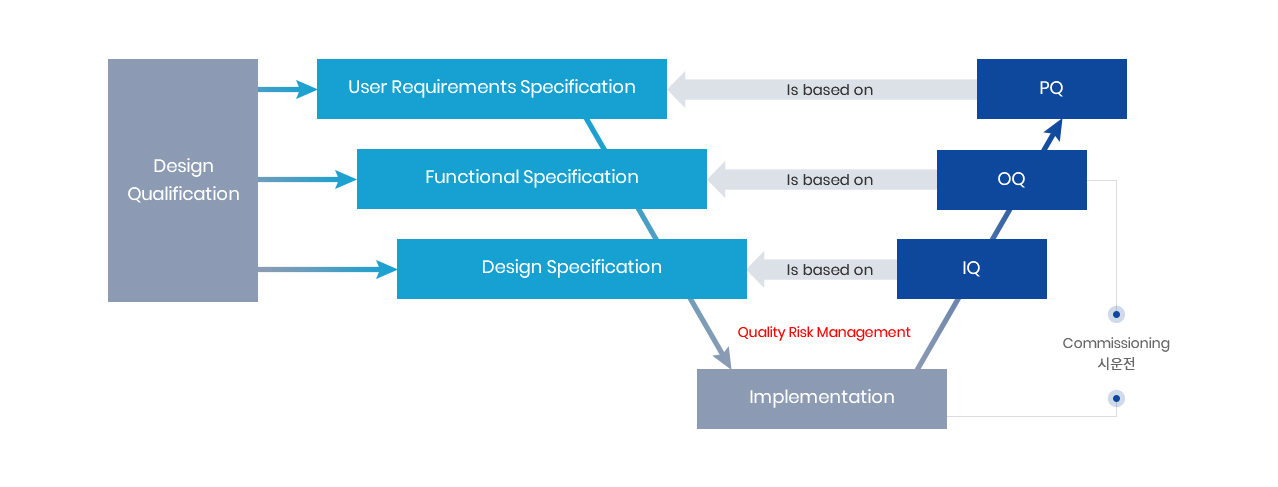
V모델은 Vessel류 등 제조 및 지원 설비에 대한 밸리데이션 순서를 관리하는데 주로 사용된다.
V모델에서는 설계적격성평가(DQ)의 문서와 설치, 운전(OQ) 및 성능 적격성평가(PQ)의 프로토콜과 연결시켜 놓았다.
Vessel류는 제품에 직접적인 영향을 미치기도 하고, 일부 Vessel은 공정의 보조적인 역할을 하기도 한다.
적격성평가를 할 것인지 단지 시운전만 수행할 것인지에 대한 결정은 그 사용 용도에 따라 다르다. 위험평가는 이러한 결정을 하는데 활용할 수 있다.
대표적으로 적격성 평가가 필요한 Vessel류는 다음과 같다.